Publications
First-author publications by graduate students
1. Tani H, Miyamoto R, Nagashima T, Michishita M, Tamura K, Bonkobara M. Molecular characterization of canine SHP2 mutants and anti-tumour effect of SHP2 inhibitor, SHP099, in a xenograft mouse model of canine histiocytic sarcoma. Vet Comp Oncol. 2022. 20(1):109-117. doi: 10.1111/vco.12751.
2. Tani H, Miyamoto R, Miyazaki T, Oniki S, Tamura K, Bonkobara M. A feline case of multiple myeloma treated with bortezomib. BMC Vet Res. 2022. 18(1):384. doi: 10.1186/s12917-022-03484-1.
3. Miyamoto R, Tani H, Ikeda T, Saima H, Tamura K, Bonkobara M. Commitment toward cell death by activation of autophagy with survivin inhibitor YM155 in two canine squamous cell carcinoma cell lines with high expression of survivin. Res Vet Sci. 2021. 135:412-415. doi: 10.1016/j.rvsc.2020.10.025.
4. Tani H, Miyamoto R, Noguchi S, Kurita S, Nagashima T, Michishita M, Yayoshi N, Tamura K, Bonkobara M. A canine case of malignant melanoma carrying a KIT c.1725_1733del mutation treated with toceranib: a case report and in vitro analysis. BMC Vet Res. 2021. 17(1):147. doi: 10.1186/s12917-021-02864-3.
5. Tani H, Kurita S, Miyamoto R, Ochiai K, Tamura K, Bonkobara M. Canine histiocytic sarcoma cell lines with SHP2 p.Glu76Gln or p.Glu76Ala mutations are sensitive to allosteric SHP2 inhibitor SHP099. Vet Comp Oncol. 2020. 18(2):161-168. doi: 10.1111/vco.12524.
– Selected for the journal cover –
6. Tani H, Kurita S, Miyamoto R, Sawada H, Fujiwara-Igarashi A, Michishita M, Azakami D, Hasegawa D, Tamura K, Bonkobara M. Nimustine Treatment of 11 Cases of Canine Histiocytic Sarcoma. J Am Anim Hosp Assoc. 2020. 56(3):146. doi: 10.5326/JAAHA-MS-6959.
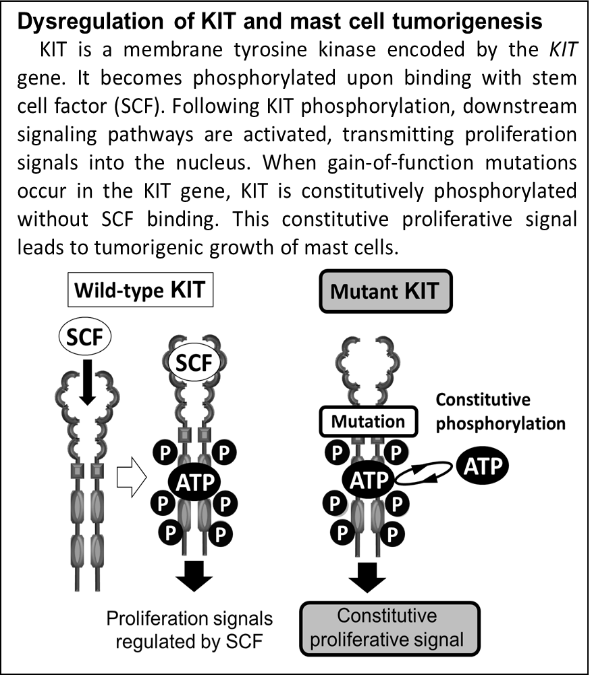
7. Kurita S, Miyamoto R, Tani H, Kobayashi M, Sasaki T, Tamura K, Bonkobara M. Genetic alterations of KIT during clonal expansion and subsequent acquisition of resistance to toceranib in a canine mast cell tumor cell line. J Vet Pharmacol Ther. 2019. 42(6):673-681. doi: 10.1111/jvp.12816.
8. Miyamoto R, Kurita S, Tani H, Ikeda T, Ishizaka M, Saima H, Kobayashi M, Tamura K, Bonkobara. Canine squamous cell carcinoma cell lines with high expression of survivin are sensitive to survivin inhibitor YM155. M. Vet J. 2018. 240:31-36. doi: 10.1016/j.tvjl.2018.09.001.
9. Miyamoto R, Kurita S, Tani H, Kobayashi M, Sugiura S, Shigihara K, Sato Y, Tanaka Y, Tamura K, Bonkobara M. Establishment and characterization of a cell line from a feline histiocytic sarcoma. Vet Immunol Immunopathol. 2018. 201:72-76. doi: 10.1016/j.vetimm.2018.05.011.
10. Ito K, Miyamoto R, Tani H, Kurita S, Kobayashi M, Tamura K, Bonkobara M. Effect of dasatinib in a xenograft mouse model of canine histiocytic sarcoma and in vitro expression status of its potential target EPHA2. J Vet Pharmacol Ther. 2018. 41(1):e45-e48. doi: 10.1111/jvp.12449. Epub 2017 Aug 17.
11. Kobayashi M, Kuroki S, Kurita S, Miyamoto R, Tani H, Tamura K, Bonkobara M. A decrease in ubiquitination and resulting prolonged life-span of KIT underlies the KIT overexpression-mediated imatinib resistance of KIT mutation-driven canine mast cell tumor cells. Oncol Rep. 2017. 38(4):2543-2550. doi: 10.3892/or.2017.5865.
12. Kuroki S, Kobayashi M, Tani H, Miyamoto R, Kurita S, Tamura K, Ono K, Washizu T, Bonkobara M. Selective growth inhibition by suppression of F1Fo ATPase in canine malignant melanoma cell lines. J Vet Pharmacol Ther. 2017. 40(1):101-104. doi: 10.1111/jvp.12336.
13. Kobayashi M, Kuroki S, Tanaka Y, Moriya Y, Kozutumi Y, Uehara Y, Ono K, Tamura K, Washizu T, Bonkobara M. Molecular changes associated with the development of resistance to imatinib in an imatinib-sensitive canine neoplastic mast cell line carrying a KIT c.1523A>T mutation. Eur J Haematol. 2015. 95(6):524-31. doi: 10.1111/ejh.12526.
14. Ito K, Kobayashi M, Kuroki S, Sasaki Y, Iwata T, Mori K, Kuroki T, Ozawa Y, Tetsuka M, Nakagawa T, Hiroi T, Yamamoto H, Ono K, Washizu T, Bonkobara M. The proteasome inhibitor bortezomib inhibits the growth of canine malignant melanoma cells in vitro and in vivo. Vet J. 2013. 198(3):577-82. doi: 10.1016/j.tvjl.2013.08.003.
15. Kobayashi M, Kuroki S, Ito K, Yasuda A, Sawada H, Ono K, Washizu T, Bonkobara M. Imatinib-associated tumour response in a dog with a non-resectable gastrointestinal stromal tumour harbouring a c-kit exon 11 deletion mutation. Vet J. 2013. 198(1):271-4. doi: 10.1016/j.tvjl.2013.05.035.
16. Ito K, Kuroki S, Kobayashi M, Ono K, Washizu T, Bonkobara M. Identification of dasatinib as an in vitro potent growth inhibitor of canine histiocytic sarcoma cells. Vet J. 2013. 196(3):536-40. doi: 10.1016/j.tvjl.2012.12.016.
17. Kobayashi M, Sugisaki O, Ishii N, Yamada O, Ito K, Kuroki S, Sasaki Y, Ono K, Washizu T, Bonkobara M. Canine intestinal mast cell tumor with c-kit exon 8 mutation responsive to imatinib therapy. Vet J. 2012. 193(1):264-7. doi: 10.1016/j.tvjl.2011.10.027.
Review・Editorial
18. Bonkobara M. Recent developments in veterinary diagnostics: Current status and future potential. Vet J. 2016. 215:1-2. doi: 10.1016/j.tvjl.2016.08.010.
19. Takeuchi Y, Bonkobara M. Receptor tyrosine kinase KIT: Prognostic and therapeutic involvement in canine mast cell tumours. Vet J. 2016. 210:5-6. doi: 10.1016/j.tvjl.2015.07.012.
20. Bonkobara M. Dysregulation of tyrosine kinases and use of imatinib in small animal practice. Vet J. 2015. 205(2):180-8. doi: 10.1016/j.tvjl.2014.12.015.
Editor
21. Bonkobara M. Editor of Special Issue: Recent Developments in Veterinary Diagnostics. The Veterinary Journal. 2016. Vol 215.
Collaborative research
22. Kobayashi M, Onozawa M, Watanabe S, Nagashima T, Tamura K, Kubo Y, Ikeda A, Ochiai K, Michishita M, Bonkobara M, Kobayashi M, Hori T, Kawakami E. Establishment of a BRAF V595E-mutant canine prostate cancer cell line and the antitumor effects of MEK inhibitors against canine prostate cancer. Vet Comp Oncol. 2023. doi: 10.1111/vco.12879.
23. Maeda S, Nakazawa M, Uchida M, Yoshitake R, Nakagawa T, Nishimura R, Miyamoto R, Bonkobara M, Yonezawa T, Momoi Y. Foxp3+ Regulatory T Cells Associated With CCL17/CCR4 Expression in Carcinomas of Dogs. Vet Pathol. 2020. 57(4):497-506. doi: 10.1177/0300985820921535.
24. Gentilini F, Turba ME, Dally C, Takanosu M, Kurita S, Bonkobara M. The secondary KIT mutation p.Ala510Val in a cutaneous mast cell tumour carrying the activating mutation p.Asn508Ile confers resistance to masitinib in dogs. BMC Vet Res. 2020. 19;16(1):64. doi: 10.1186/s12917-020-02284-9.
25. Igase M, Shousu K, Fujiki N, Sakurai M, Bonkobara M, Hwang CC, Coffey M, Noguchi S, Nemoto Y, Mizuno T. Anti-tumour activity of oncolytic reovirus against canine histiocytic sarcoma cells. Vet Comp Oncol. 2019. 17(2):184-193. doi: 10.1111/vco.12468.
26. Nakano Y, Kobayashi M, Bonkobara M, Takanosu M. Identification of a secondary mutation in the KIT kinase domain correlated with imatinib-resistance in a canine mast cell tumor. Vet Immunol Immunopathol. 2017. 188:84-88. doi: 10.1016/j.vetimm.2017.05.004.
27. Kato Y, Ochiai K, Kawakami S, Nakao N, Azakami D, Bonkobara M, Michishita M, Morimatsu M, Watanabe M, Omi T. Canine REIC/Dkk-3 interacts with SGTA and restores androgen receptor signalling in androgen-independent prostate cancer cell lines. BMC Vet Res. 2017. 13(1):170. doi: 10.1186/s12917-017-1094-4.
28. Kobayashi M, Saito A, Tanaka Y, Michishita M, Kobayashi M, Irimajiri M, Kaneda T, Ochiai K, Bonkobara M, Takahashi K, Hori T, Kawakami E. MicroRNA expression profiling in canine prostate cancer. J Vet Med Sci. 2017. 79(4):719-725. doi: 10.1292/jvms.16-0279.
29. Nakahira R, Michishita M, Kato M, Okuno Y, Hatakeyama H, Yoshimura H, Azakami D, Ochiai K, Bonkobara M, Takahashi K. Oncocytic carcinoma of the salivary gland in a dog. J Vet Diagn Invest. 2017. 29(1):105-108. doi: 10.1177/1040638716673126.
30. Omi T, Nakazawa S, Udagawa C, Tada N, Ochiai K, Chong YH, Kato Y, Mitsui H, Gin A, Oda H, Azakami D, Tamura K, Sako T, Inagaki T, Sakamoto A, Tsutsui T, Bonkobara M, Tsuchida S, Ikemoto S. Molecular Characterization of the Cytidine Monophosphate-N-Acetylneuraminic Acid Hydroxylase (CMAH) Gene Associated with the Feline AB Blood Group System. PLoS One. 2016. 11(10):e0165000. doi: 10.1371/journal.pone.0165000.
31. Ochiai K, Oda H, Shono S, Kato Y, Sugihara S, Nakazawa S, Azakami D, Michishita M, Onozawa E, Bonkobara M, Sako T, Shun-Ai L, Ueki H, Watanabe M, Omi T. Properties of the feline tumour suppressor reduced expression in immortalized cells (REIC/Dkk-3). Vet Comp Oncol. 2017. 15(4):1181-1186. doi: 10.1111/vco.12254.
32. Azakami D, Nakahira R, Kato Y, Michishita M, Kobayashi M, Onozawa E, Bonkobara M, Kobayashi M, Takahashi K, Watanabe M, Ishioka K, Sako T, Ochiai K, Omi T. The canine prostate cancer cell line CHP-1 shows over-expression of the co-chaperone small glutamine-rich tetratricopeptide repeat-containing protein α. Vet Comp Oncol. 2017. 15(2):557-562. doi: 10.1111/vco.12199.
33. Kato Y, Ochiai K, Michishita M, Azakami D, Nakahira R, Morimatsu M, Ishiguro-Oonuma T, Yoshikawa Y, Kobayashi M, Bonkobara M, Kobayashi M, Takahashi K, Watanabe M, Omi T. Molecular cloning of canine co-chaperone small glutamine-rich tetratricopeptide repeat-containing protein α (SGTA) and investigation of its ability to suppress androgen receptor signalling in androgen-independent prostate cancer. Vet J. 2015. 206(2):143-8. doi: 10.1016/j.tvjl.2015.08.002.
34. Ochiai K, Ishiguro-Oonuma T, Yoshikawa Y, Udagawa C, Kato Y, Watanabe M, Bonkobara M, Morimatsu M, Omi T. Polymorphisms of canine BRCA2 BRC repeats affecting interaction with RAD51. Biomed Res. 2015. 36(2):155-8. doi: 10.2220/biomedres.36.155.